Type I Collagen Suspension Induces Neocollagenesis and Myodifferentiation in Fibroblasts In Vitro
The ability of a collagen-based matrix to support cell proliferation, migration, and infiltration has been reported; however, the direct effect of an aqueous collagen suspension on cell cultures has not been studied yet. In this work, the effects of a high-concentration aqueous suspension of a micronized type I equine collagen (EC-I) have been evaluated on a normal mouse fibroblast cell line.
Francesca Lombardi, Paola Palumbo, Francesca Rosaria Augello, Ilaria Giusti, Vincenza Dolo, Luca Guerrini, Maria Grazia Cifone, Maurizio Giuliani and Benedetta Cinque.
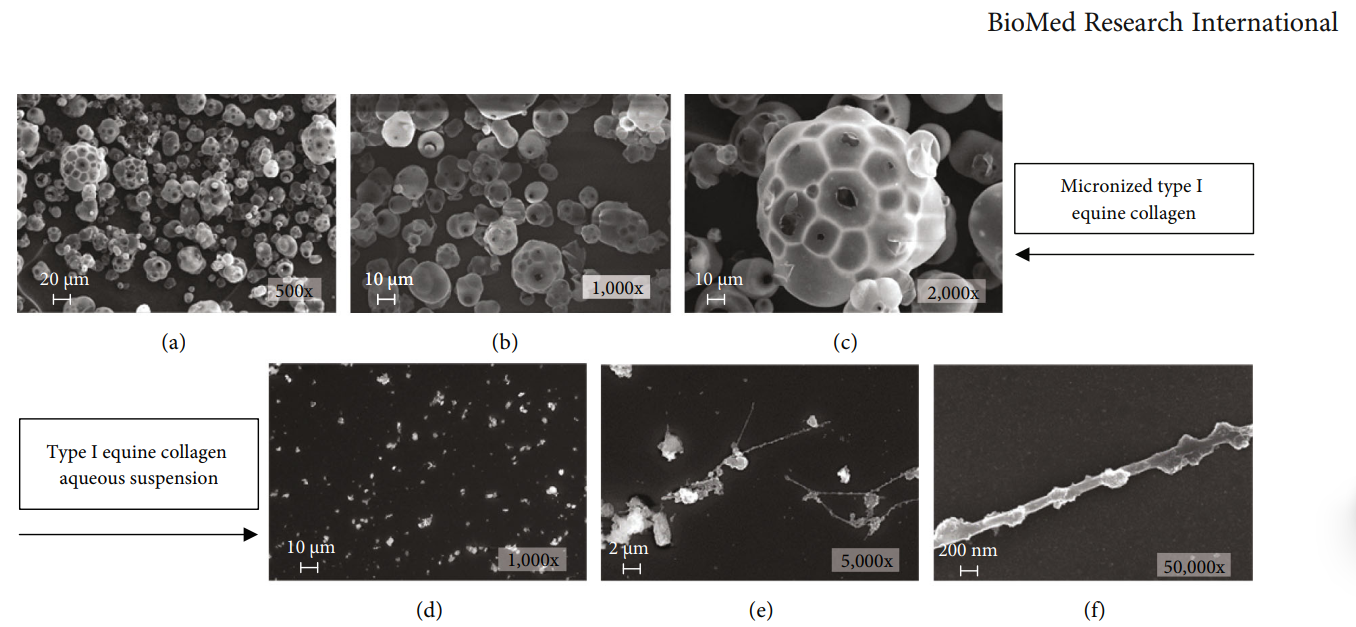
The ability of a collagen-based matrix to support cell proliferation, migration, and infiltration has been reported; however, the direct effect of an aqueous collagen suspension on cell cultures has not been studied yet. In this work, the effects of a high-concentration aqueous suspension of a micronized type I equine collagen (EC-I) have been evaluated on a normal mouse fibroblast cell line. Immunofluorescence analysis showed the ability of EC-I to induce a significant increase of type I and III collagen levels, parallel with overexpression of crucial proteins in collagen biosynthesis, maturation, and secretion, prolyl 4-hydroxylase (P4H) and heat shock protein 47 (HSP47), as demonstrated by western blot experiments. The treatment led, also, to an increase of α-smooth muscle actin (α-SMA) expression, evaluated through western blot analysis, and cytoskeletal reorganization, as assessed by phalloidin staining. Moreover, scanning electron microscopy analysis highlighted the appearance of plasma membrane extensions and blebbing of extracellular vesicles. Altogether, these results strongly suggest that an aqueous collagen type I suspension is able to induce fibroblast myodifferentiation. Moreover, our findings also support in vitro models as a useful tool to evaluate the effects of a collagen suspension and understand the molecular signaling pathways possibly involved in the effects observed following collagen treatment in vivo.
Collagen is the most abundant protein in the human body, where it is responsible for structure, stability, and strength, especially within the dermal layers [1]. Alterations in the structure and quantity of collagen fibers are the main changes observed in aged skin [2, 3]. In fact, while in young skin collagen fibrils are intact, abundant, tightly packed, and well-organized, with advancing age, they undergo progressive loss and fragmentation, causing the atrophy and collapse of skin tissue and resulting in skin wrinkling and thinning, loss of elasticity, drying, and roughing [4–6]. An increase of collagen degradation together with the reduction of its biosynthesis is implicated in altered collagen homeostasis, which results in a net collagen deficiency [7–9]. Previous studies show that skin fibroblasts isolated from older people have altered migration, premature senescence, reduced proliferative response, and defects in matrix generation compared with dermal fibroblasts of young people [10–13]. In youthful skin, fibroblasts attach to the surrounding intact extracellular matrix (ECM). This attachment allows fibroblasts to exert mechanical forces on the surrounding ECM and to spread and maintain a standard elongated shape [2].

Xem thêm